TALLAHASSEE, Fla. — The governor was still hopeful Tuesday that a COVID-19 vaccine by Johnson & Johnson will be key to expanding shots beyond Florida's seniors. That's despite new phase-three trial research showing lower than anticipated efficacy.
SPECIAL COVERAGE: Coronavirus
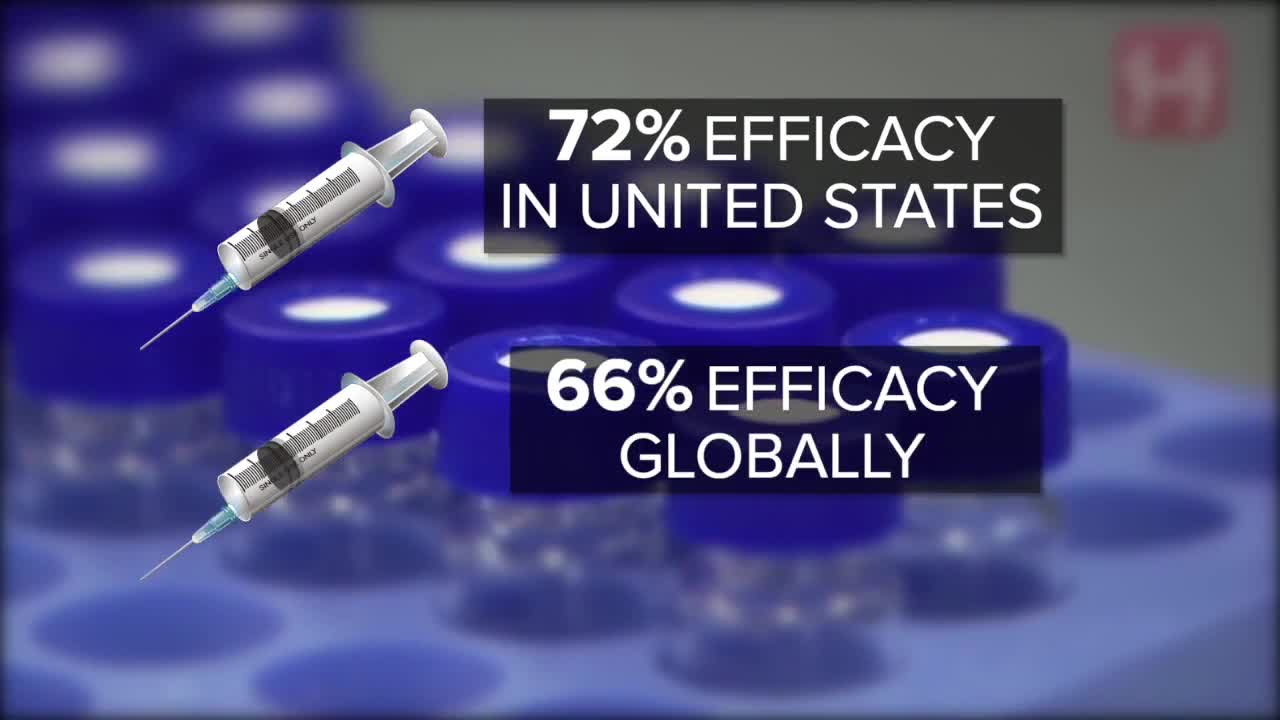
Released last weekend, the data suggested J&J's single shots were 72% effective in the U.S. and 66% effective globally. Versions from Pfizer and Moderna have near 95% efficacy, requiring two doses over several weeks.
The upside is that researchers did find J&J's vaccine was very effective at preventing death and hospitalizations, which Gov. DeSantis seized on.
"This is remarkable good news," DeSantis said during a news conference. "This is tremendous news."

When asked, the Florida Republican said he was also open to the idea of triaging the vaccine supply, setting aside Pfizer and Moderna for the most vulnerable, then using Johnson and Johnson shots to inoculate those less at risk of serious illness.
"Once we figure out how much we're getting -- however much the feds send us -- then we can make determinations about the best way to use it," DeSantis said. "There may be some folks who are seniors who prefer J&J. Who knows?"

Johnson & Johnson was expecting to file for emergency use authorization by the Food and Drug Administration in the coming days. The review process will likely take weeks.
Epidemiologists encourage virus protections to continue in the meantime.
Florida International Professor Mary Jo Trepka said less spread would mean fewer mutations, something all vaccines have shown at least some weakness to depending on the strain.
"We're kind of in a race here against the virus," Trepka said. "The way that we can beat the virus is staying ahead of it and keeping transmission rates low."