Two hundred million dollars – that's how much money Monat Global says it made selling hair care products in 2017 through multi-level marketing.
YouTube videos show the family behind Monat in mansions and driving fancy cars — but an ongoing class action lawsuit claims the company is nothing more than a pyramid scheme.
Three recently filed class action lawsuits accuse Monat of fraud and deception.
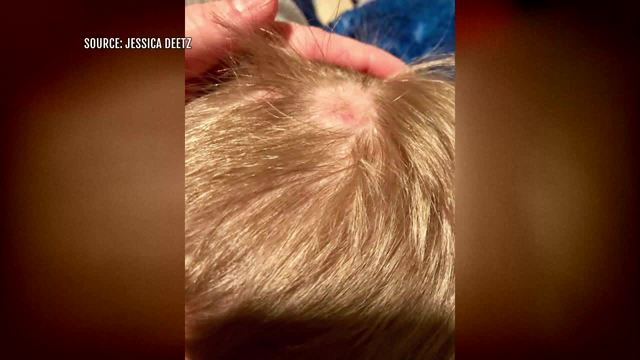
"An inherent design and/or manufacturing defect in Monat hair care products causes significant hair loss and scalp irritation to many consumers," one lawsuit alleges.
"I'm devastated with what my hair looks like right now," said Heather Fox, a Monat customer in Phoenix.
"I had to cut off my hair," said Monat Market Partner Erin Ostby.
Both women say Monat did them more damage than good.
"My dad used to always say I was like Samson from the Bible – I got my power from my hair. So it was really upsetting and I couldn't do that to someone else," said Ostby, who recently stopped selling Monat.
CLICK TO SEE THE ORIGINAL COMPLAINTS
The U.S. Food and Drug Administration has received and is in the process of assessing 187 adverse event reports related to Monat products. The FDA received these reports between Aug. 2, 2017 8/27/17 and March 9, 2018.
More than 500 complaints have been filed with the Better Business Bureau in South Florida, where Monat is headquartered.
"Any reputable lab will tell you there's nothing in the products that would cause this kind of reaction in a large population – there just isn't. You can rub it in your skin, you can drink it if you like, within reason. It's not going to cause this kind of reaction," Monat Spokesperson Gene Grabowski said in a phone interview.
But the company's repeated response to BBB complaints states that, "although Monat's ingredients are naturally-based, safe, pure and sustainable, we understand that some may experience a reaction and should discontinue use."
The class action lawsuits claim the products use numerous "harsh chemicals" and "known human allergens."
One controversial ingredient the suit highlights is Capixyl – containing red clover – which some say should be on a warning label.
"Yes, there should be a warning label, but only if the amount was higher," Grabowski said.
The University of Maryland Medical Center says women with a history of breast cancer should avoid red clover due to its estrogen-like effects in the body. It also says red clover may interfere with the liver's ability to process some drugs.
"The amount of red clover used (red clover extract) is so small that it would have no effect," said Grabowski.
According to the FDA, cosmetic companies are responsible for ensuring the safety of their own products, which in most cases don't require government approval before they go on the market.
"We do tests before we send them to market and we know they're safe," said Grabowski. "I mean, those aren't clinical tests."
Monat's website shows the clinical tests they did utilized one active ingredient per study.
As the class action lawsuits were recently filed, Monat has not yet responded to the claims in court.